When a patient switches from a brand-name HIV drug to a generic version, most doctors assume it’s a simple swap-same active ingredient, same effect. But for drugs with a narrow therapeutic index, that assumption can be dangerous. Therapeutic drug monitoring isn’t just a lab test-it’s a safety net for patients on generic versions of drugs where tiny changes in blood levels can mean the difference between treatment success and failure.
What makes a drug a narrow therapeutic index (NTI) drug?
NTI drugs are the kind where the gap between a helpful dose and a harmful one is razor-thin. A little too much, and you risk liver damage or nerve toxicity. A little too little, and the virus starts replicating again. For HIV treatment, this applies mainly to protease inhibitors (like lopinavir and atazanavir) and some non-nucleoside reverse transcriptase inhibitors (like efavirenz and nevirapine). These aren’t the same as NRTIs-the "nukes" like tenofovir or emtricitabine-which don’t need TDM because they work inside cells, not in the bloodstream.
Generic versions of these drugs are chemically identical on paper. But in real life, differences in how they’re made-fillers, coating, absorption rates-can change how much actually gets into the blood. That’s where TDM comes in. It measures the actual drug concentration in a patient’s plasma, not just what the pill bottle says.
Why TDM matters more with generics
In the UK, the NHS has been using generic antiretrovirals for over a decade to cut costs. But in 2022, an internal audit of 147 patients on generic lopinavir/ritonavir found that 12% had drug levels outside the safe range. Some were dangerously high-leading to nausea, dizziness, even jaundice. Others were too low, with viral loads creeping back up despite perfect adherence.
One NHS clinic in Bristol reported a case where a patient on generic lopinavir had undetectable viral load for months, then suddenly jumped to 1,200 copies/mL. No missed doses. No new meds. TDM showed his lopinavir concentration had dropped by 40%-a batch variation in the generic formulation had slowed absorption. Adjusting the dose brought the level back up, and the virus vanished again within six weeks.
This isn’t rare. Studies from South Africa show that when TDM is used with generic antiretrovirals in resource-limited settings, treatment failure drops by 22%. That’s not just a statistic-it’s people staying alive.
Who actually needs TDM?
TDM isn’t for everyone. The UK HIV guidelines say it’s only recommended in specific situations:
- Patients switching to a new generic version of a protease inhibitor or NNRTI
- Those with liver or kidney damage affecting drug clearance
- People on TB treatment (rifampicin or rifapentine can slash drug levels by up to 30%)
- Children, where dosing is based on weight and metabolism changes fast
- Anyone with unexplained treatment failure or toxicity
For most people on stable, brand-name regimens with no interactions, TDM adds cost without benefit. But for those on generics in complex situations? It’s not optional-it’s essential.
The hidden costs and delays
Each TDM test in the UK NHS costs between £250 and £350. That’s not cheap. And results? They take 10 to 14 days. In a system where viral load tests give answers in 3-5 days, waiting two weeks for TDM feels like a step backward.
One patient on Reddit described waiting six weeks for results-by then, his viral load had spiked, and he’d already started developing resistance. "I felt like the system was testing me, not helping me," he wrote.
Private labs in the US offer faster turnaround-2 to 3 days-for around $450-$650. But in the NHS, there are only three to five labs that can run these tests reliably. Most clinics don’t even know how to order them.
How TDM works in practice
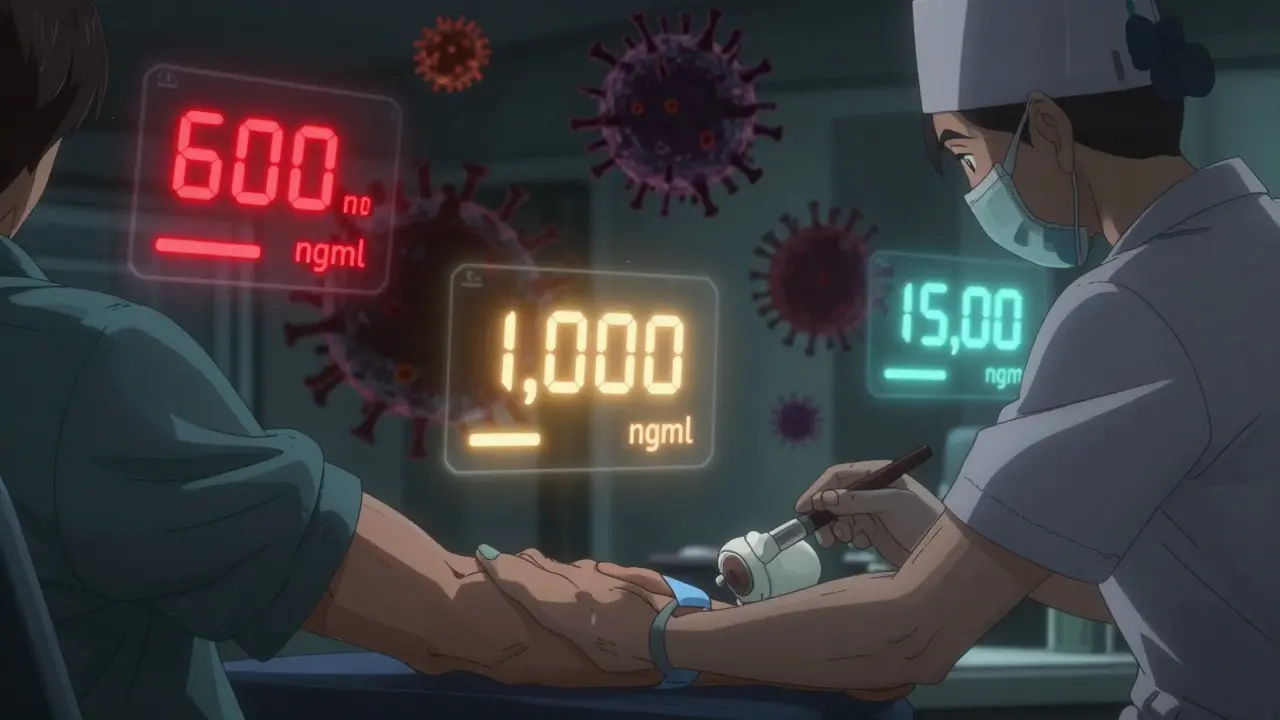
It’s not just about drawing blood. The timing matters. Trough levels-the lowest concentration before the next dose-are what clinicians look at. For lopinavir, the target is 1,000 ng/mL or higher. Below 600 ng/mL? Risk of resistance. Above 15,000 ng/mL? Risk of liver damage.
But here’s the catch: those numbers aren’t set in stone. Different labs use different assays. What one lab calls "low," another calls "normal." That’s why only accredited labs with ISO 15189 certification should run these tests. And why results need to be interpreted by someone who knows HIV pharmacology-not just a lab tech.
At McGill University Health Centre in Canada, infectious disease specialists review every TDM result with the prescribing clinician. They adjust doses based on weight, kidney function, and other meds. That kind of teamwork is what makes TDM work.
What’s holding TDM back?
There are three big barriers:
- Cost and access-Only a handful of UK labs can do it, and insurance rarely covers it unless it’s "medically necessary."
- Lack of training-Most GPs and even some HIV specialists don’t understand pharmacokinetics. They don’t know how to interpret a Cmin value.
- No universal targets-There’s no single agreed-upon "safe" level for every drug. Guidelines vary. Studies are still ongoing.
The European AIDS Clinical Society (EACS) says TDM should only be used selectively. The US HHS guidelines don’t recommend it routinely. But both agree: when you’re dealing with generics and complex drug interactions, it’s one of the few tools you’ve got.
What’s changing?
More new HIV drugs are being developed-like lenacapavir and islatravir-that are NTI drugs too. As these roll out, TDM will become even more important. And with global efforts to expand access to generics in low-income countries, TDM is being piloted in places like Kenya and Malawi to ensure these cheaper drugs aren’t failing silently.
The biggest shift? TDM is no longer seen as a research tool. It’s becoming a clinical safety protocol-for specific patients, in specific situations. It’s not about replacing viral load tests. It’s about working alongside them.
Bottom line: TDM isn’t magic. But it’s necessary.
Therapeutic drug monitoring won’t fix poor adherence. It won’t replace good patient education. But when you’re giving someone a generic version of a drug where 10% less in the blood can lead to drug resistance, you can’t afford to guess.
For patients on generic protease inhibitors or NNRTIs-especially those with liver disease, TB co-infection, or who’ve had treatment failure-TDM isn’t a luxury. It’s a lifeline. The data is clear: when used correctly, it reduces failure rates by 15-20%. It prevents toxicity. It saves lives.
The question isn’t whether TDM works. It’s whether we’re willing to make it accessible to the people who need it most.
Is therapeutic drug monitoring used for all HIV drugs?
No. TDM is only used for drugs with a narrow therapeutic index that circulate in the bloodstream-mainly protease inhibitors and some NNRTIs. It’s not used for NRTIs (like tenofovir or emtricitabine) because they’re prodrugs that become active inside cells, so their blood levels don’t reflect their effect.
How accurate are TDM tests for generic drugs?
Accuracy depends on the lab. Only labs with ISO 15189 accreditation use validated assays that can detect small differences between brand and generic formulations. Unaccredited labs may miss variations that could lead to under- or overdosing.
Can TDM replace viral load testing?
No. Viral load testing tells you if the virus is under control. TDM tells you if the drug level in your blood is high enough to keep it there. They work together. A patient can have a normal viral load but low drug levels-meaning they’re one missed dose away from failure. Or they can have high drug levels and no virus, but risk toxicity.
Why isn’t TDM used more widely in the UK?
Cost, limited lab capacity, and lack of clinician training are the main reasons. Only 3-5 NHS labs can run these tests reliably, and results take up to two weeks. Most clinics don’t have the expertise to interpret the results, and funding isn’t guaranteed unless specific criteria are met.
Does TDM help with drug interactions?
Yes, especially with tuberculosis medications like rifampicin or rifapentine, which can cut HIV drug levels by 25-30%. TDM lets clinicians adjust doses before resistance develops. In one JAMA study, patients on dolutegravir and rifapentine had their doses adjusted based on TDM-resulting in 97.7% achieving undetectable viral load at 48 weeks.
Are there risks to using TDM?
Yes. The biggest risk is relying on TDM instead of addressing the root cause-like poor adherence or malabsorption. TDM doesn’t fix those. It just tells you something’s wrong. Also, if results are delayed or misinterpreted, it can lead to unnecessary dose changes that cause harm.
What’s the future of TDM for HIV treatment?
TDM is becoming more targeted-not routine, but used for high-risk patients: those on generics, with organ damage, on TB meds, or in pediatric care. As new NTI drugs enter the market, and global access to generics expands, TDM will be critical to ensure safety. Research is also moving toward point-of-care testing, which could cut turnaround time to hours instead of weeks.

15 Comments
January 31, 2026 Bryan Coleman
Just had a patient switch to generic lopinavir last month. TDM showed his levels were half what they should be. No missed doses, no new meds. Just bad batch variation. We adjusted and he’s undetectable again. This isn’t theoretical-it’s happening right now.
February 2, 2026 Sami Sahil
India is starting to use TDM in big govt clinics now. We had a guy on generic efavirenz with viral rebound-turns out his generic had a different coating. Took 3 weeks to get results but it saved his treatment. TDM = lifesaver when you got no choice but generics.
February 3, 2026 franklin hillary
Let me be real for a sec. We’re letting pharmaceutical companies play Russian roulette with people’s lives because it’s cheaper. You think a pill is a pill? Nah. It’s a lottery ticket with your immune system as the prize. TDM isn’t fancy tech-it’s basic damn decency. And if your system can’t afford to test blood levels before someone develops resistance, then your system is broken.
February 3, 2026 Rachel Liew
I’m a nurse in a rural clinic and we don’t even have access to TDM. I’ve seen patients panic when their viral load spikes and they swear they didn’t miss a pill. It breaks my heart. We need this to be standard-not a luxury. Please don’t let cost be the reason someone loses their treatment.
February 5, 2026 Nicki Aries
There’s a systemic failure here. Not just in the NHS, but globally. We have the science. We have the data. We have the tools. But we’re still treating life-saving diagnostics like optional add-ons. That’s not policy-it’s negligence. And people are dying because of it. We need to stop pretending this is about budgets and start admitting it’s about priorities.
February 5, 2026 Ed Di Cristofaro
Oh please. People are just lazy. If you can’t take your meds right, why should we waste money on fancy blood tests? Just get your act together. TDM is a crutch for noncompliant patients.
February 7, 2026 Bob Cohen
So let me get this straight-we’re spending $300 every two weeks to check if a pill worked… but we won’t spend $50 to educate patients on how to take them? The real problem isn’t the generic. It’s the lack of support. TDM is a band-aid on a gunshot wound.
February 8, 2026 Ishmael brown
Who really controls the generic labs? 🤔 I’ve heard whispers that some manufacturers tweak formulations just enough to pass regulatory tests but still mess with absorption. TDM isn’t about safety-it’s about exposing corporate fraud. And no one wants that exposed.
February 9, 2026 Aditya Gupta
My cousin in Kenya got generic lopinavir. No TDM. Viral load jumped. Got switched back to brand. Took 4 months. He almost lost his job. TDM should be free for everyone on generics. No excuses.
February 10, 2026 Nancy Nino
While I appreciate the clinical rationale presented, one must consider the opportunity cost of allocating finite healthcare resources toward TDM when scalable, evidence-based adherence interventions remain underfunded and underutilized.
February 12, 2026 Jaden Green
Look, I’ve read the guidelines. I’ve seen the papers. But let’s be honest-this is just another way for big pharma to justify their profit margins. The real issue? The FDA and EMA let generics slide through with minimal bioequivalence testing. TDM is just damage control for a regulatory failure they refuse to fix. And now we’re all paying for it-in blood tests, in wait times, in anxiety.
February 12, 2026 Angel Fitzpatrick
They’re not just testing drug levels-they’re testing your loyalty to the system. TDM is a surveillance tool disguised as medicine. The same labs that run these tests? They’re owned by the same conglomerates that make the brand-name drugs. You think they want you to know how much cheaper generics can be? No. They want you dependent. They want you afraid. TDM isn’t safety-it’s control.
February 12, 2026 Nidhi Rajpara
It is imperative to note that the variance in bioavailability between generic and brand-name formulations is statistically insignificant in over 90% of cases, according to the WHO’s 2021 bioequivalence meta-analysis. The emphasis on TDM may be disproportionate to the actual risk profile.
February 13, 2026 Chris & Kara Cutler
My husband is on generic atazanavir. TDM saved him. No joke. 🙏 We’re so grateful. If your clinic doesn’t offer it-ask. Demand it. It’s worth it.
February 14, 2026 Donna Macaranas
I’ve been on generic lopinavir for 5 years. Never had an issue. TDM sounds good, but maybe it’s only needed for a small group? Not everyone needs it. Don’t make it a panic.
Write a comment